Menu
Medical Device Regulation (MDR)
Ready to go for the new MDR with Amoena
The Medical Device Regulation (MDR) is a European regulation for medical devices that came into force at the end of May 2017 (EU 2017/745). After a transitional period, which was extended for coronavirus reasons, the requirements are to be applied from 26 May 2021 at the latest. For both manufacturers of medical devices and distributors, significant changes must be implemented by the deadline at the latest.
Amoena is well prepared for the application of the new Medical Device Regulation from May 26th on. In implementing the MDR requirements we have made them as practical, simple and transparent as possible for both parties – for us as a manufacturer and for our distribution partners.
What are the visible effects of the MDR?
According to the MDR, extended information is required for the labelling of medical devices. In future, in addition to the code that clearly identifies the products, you will find further data and symbols on the packaging and label. Products under the responsibility of Amoena Medizin-Orthopädie-Technik GmbH (distributor and manufacturer) can be recognised by our address behind a black factory symbol on the label or packaging:
 Amoena Medizin-Orthopädie-Technik GmbH
Amoena Medizin-Orthopädie-Technik GmbH
Kapellenweg 36, 83064 Raubling, Germany
www.amoena.com
What happens with older products (see Art. 120 para. 4 MDR)?
Products with old labels may:
- be sold by you as a specialist retailer until May 2025,
- as long as the expiry date is not exceeded before then.
- This also applies to products that arrived at your consignment warehouse before 26 May 2021. These are considered to have been placed on the market and do not need to be relabelled.
What exactly are the requirements for product labelling?
In addition to the familiar CE mark, you will also find some new symbols which we would like to introduce to you below:
Icon | Explanation |
 | Expiration date: From the date of validity of the MDR, products have to contain a manufacturing or expiry date. Our Breast Forms and Form Care products will carry an expiry date. The expiry date is determined on the basis of shelf life studies. |
 | Date of manufacture (= shipping date): From the date of validity of the MDR, products must contain a manufacturing or expiry date. Our Recovery Care and other Textiles will carry a manufacturing date. |
 | Reference number: Symbol is displayed before the article number |
 | Serial number: Symbol is displayed before the serial number |
 | Production lot number: Symbol is displayed before the production lot number |
 | The symbol "MD" stands for Medical Device – this symbol is displayed to indicate that the product is a medical device. |
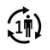 | Single patient - multiple use: The product can be used several times by one patient only. |
 | Read more information in the Instructions for use |
| | If the packaging is damaged, the product must not be used |
 | Products under the responsibility of Amoena GmbH (manufacturer) can be recognized by our address behind a black factory symbol on the label or packaging |
 | Protect the products from sunlight |
 | Store the products dry |
What about the instructions for use?
In the very unlikely event of a "serious incident" with a medical device of our risk class, the legislator obliges us to refer to a reporting obligation in the instructions for use. In addition, as required by the MDR, you and your customers will also find our current instructions for use on our homepage by the deadline at the latest.
For any further questions regarding the MDR, please do not hesitate to contact us.





